Home

Experimental reconstruction

Highly vesicular bloomery slag

Contents of one box in the National Slag Collection

Open cast iron ore mining, Romano-British

Part of an early blast furnace (Araglin, Ireland)
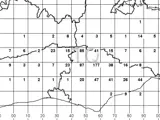
Activity frequency mapping (iron works)

Crafts

Recording artefacts in situ

Just digging

Documentary record search

Experimental Archaeology

Conferences

Artefactual evidence

More comparative studies
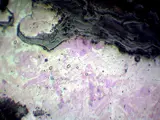
Etched sample of unworked bloomery iron (photomicrograph)

Outcrop of sideritic ore overhanging sandstone

Comparing excavated sites
